The Martini Effect
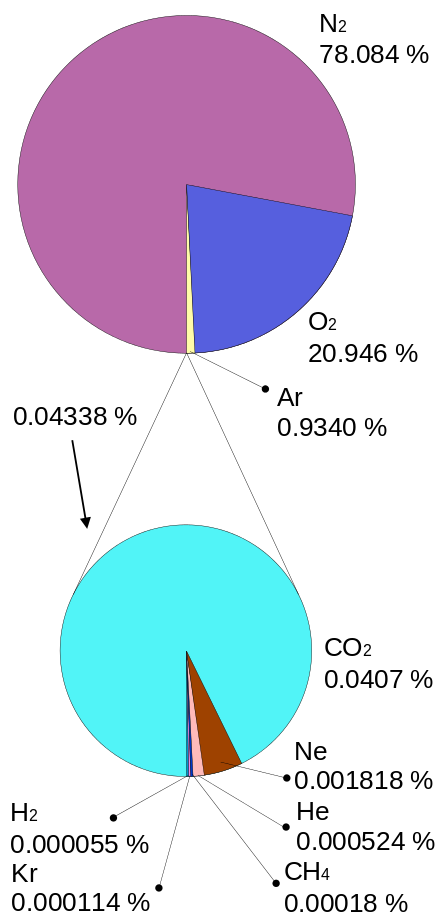 When earth cooled down after being bombarded by asteroids and comets about 4 billion years ago, earth’s atmosphere was not what it’s like today. Life evolved, and for billions of years, bacteria used sunlight to photosynthesize CO2 and water into sugars. Photosynthesis also produces a highly toxic and reactive element as a waste product: oxygen. For every sugar molecule produced, there was a tiny bacterial fart releasing 6 oxygen molecules in the atmosphere. The oxygen level in the atmosphere raised year after year until it reached a maximum of 35%, causing dragonflies to reach wingspans of 70cm. Since then oxygen levels have fluctuated to reach the level of oxygen in our atmosphere today, 20.9%. The remaining 79.1% consists almost entirely of nitrogen. The third most common element in our atmosphere is argon, at almost 1%. Number four is CO2 at 0.04% and then there are a few noble gases left, adding up to less than a percent of a percent.
When earth cooled down after being bombarded by asteroids and comets about 4 billion years ago, earth’s atmosphere was not what it’s like today. Life evolved, and for billions of years, bacteria used sunlight to photosynthesize CO2 and water into sugars. Photosynthesis also produces a highly toxic and reactive element as a waste product: oxygen. For every sugar molecule produced, there was a tiny bacterial fart releasing 6 oxygen molecules in the atmosphere. The oxygen level in the atmosphere raised year after year until it reached a maximum of 35%, causing dragonflies to reach wingspans of 70cm. Since then oxygen levels have fluctuated to reach the level of oxygen in our atmosphere today, 20.9%. The remaining 79.1% consists almost entirely of nitrogen. The third most common element in our atmosphere is argon, at almost 1%. Number four is CO2 at 0.04% and then there are a few noble gases left, adding up to less than a percent of a percent.
As scuba divers, for simplicity’s sake, we say our atmosphere contains 21% oxygen and 79% nitrogen. That’s air, that’s what you breathe. Go ahead, breathe in. Now breathe back out. From the air you breathed in, your lungs filtered out some of the oxygen and gave off CO2 in return. We need that oxygen as fuel for our internal combustion and give off CO2 as of waste product. What you breathe back out contains 16% oxygen – still enough to keep someone alive, which is why we do rescue breaths -, 5% CO2 and 79% nitrogen. Again, for simplicity’s sake. Did you notice how all the nitrogen that went in, came back out? That’s right, nothing happens inside our bodies with the nitrogen we breathe in. This changes when we go scuba diving. For regular, recreational scuba diving, we fill our scuba tanks with air. Normal, dry air. Don’t call it your oxygen tank, it’s not. It’s an air tank. When we go deeper and the surrounding pressure increases, things start to change. Actually, two things happen, regarding the nitrogen.
First, some of the nitrogen molecules you breathe in, decide they like hanging out inside of you and vote to stay around. They dissolve into your blood and travel all outskirts of your body, slowly saturating it with nitrogen. The deeper you go and the longer you dive, the more nitrogen you will absorb. Don’t be alarmed though, this does not have to be a problem if you dive within the limits and guidelines you’ve learned in your dive course. What happens if you don’t is something for another day.
The second thing nitrogen decides to do is to start playing mind games. If you descend beyond 30m depth, nitrogen starts to work on your central nervous system and begins to act like a narcotic. We call this nitrogen narcosis. Or at least we used to. Now we refer to it as gas narcosis, because in recent years, scientists discovered that other gases have a similar narcotic effect. People often compare this effect to being drunk. Which is why sometimes it’s referred to as the Martini effect. You get overconfident and euphoric, think slower, move more sluggishly, and might even start hallucinating. How much it affects you is hard to predict and will differ from day to day. With frequent exposure, you can become habituated to the effects, which enables you to venture deeper without being intoxicated. The deeper you go, the bigger the effect will be. There are stories of hallucinated pirates on a deep wreck dive. Or people that were so narked that they offered their mouthpieces to passing fish. At around 90m depth consciousness is lost. Keep in mind the limit for recreational diving is set at 40m depth.

A lot of what we know about gas narcosis was discovered by the scientist J.B.S. Haldane. In 1941, he studied the effects of nitrogen intoxication using a high-pressure chamber. His guinea pigs, who included himself and his future wife, were assigned arithmetic and manual dexterity tests. When breathing air at a pressure of 10 atmospheres, equivalent to a depth of 90m, all of them became intoxicated. One test subject, a responsible scientist at atmospheric pressure, cheated in the dexterity test. Another one alternated between depression and euphoria, one moment begging to be decompressed and the next minute laughing and attempting to mess with his colleague’s dexterity test. No one managed to get their sums right. Haldane later noted that ‘observations were not as satisfactory as could have been wished.’ Something they seemed to have overlooked was that the person administering the test was usually as intoxicated as his subjects, and often failed to take proper notes or forgot to stop the stopwatch. Such studies sufficed to show that divers suffering from gas narcosis could not be expected to behave responsibly and might react in ways that threatened their own or others’ lives.
Recovery from nitrogen poisoning occurs very quickly on decompression. In Haldane’s experiments, immediate relief of the symptoms occurred when the pressure was reduced from 10 to 5 atmospheres. The typical reaction was, ‘My God, I’m sober.’ In diving, it often suffices to ascend a few meters and the effects start to wear off.
What was this article worth to you?
![]()
